Nápady Atom According To Quantum Mechanics
Nápady Atom According To Quantum Mechanics. The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model is based on mathematics.
Tady Quantum Mechanical Atomic Model Read Physics Ck 12 Foundation
According to quantum mechanics, each electron in an atom is described by___different quantum numbers. The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. What is an atom according to quantum mechanics? The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.The more you know about one, the less you are sure about the other quantity.
What is an atom according to quantum mechanics? It is not real because you cannot do anyth. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. The more you know about one, the less you are sure about the other quantity. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. What is an atom according to quantum mechanics?

The bohr model and the quantum mechanical model... According to quantum mechanics, each electron in an atom is described by___different quantum numbers. The more you know about one, the less you are sure about the other quantity. What is an atom according to quantum mechanics?. According to quantum mechanics, each electron in an atom is described by___different quantum numbers.

A model is useful because it helps you … Two models of atomic structure are in use today: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms.

Introduction to the quantum mechanical model of the atom: 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. The quantum mechanical model is based on mathematics.. Introduction to the quantum mechanical model of the atom:

What is an atom according to quantum mechanics?. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. What is an atom according to quantum mechanics? 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron.. What is an atom according to quantum mechanics?

What is an atom according to quantum mechanics? 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. What is an atom according to quantum mechanics? For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... . For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion.

According to quantum mechanics, each electron in an atom is described by___different quantum numbers. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron.. A model is useful because it helps you …

The bohr model and the quantum mechanical model. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

The bohr model and the quantum mechanical model. Two models of atomic structure are in use today: According to quantum mechanics, each electron in an atom is described by___different quantum numbers. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed.. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron.

A model is useful because it helps you ….. So with that said, i would like to know if that … • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. What is an atom according to quantum mechanics? The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. The bohr model and the quantum mechanical model.

The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron... For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. The bohr model and the quantum mechanical model. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. A model is useful because it helps you … What is an atom according to quantum mechanics? So with that said, i would like to know if that … The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. Introduction to the quantum mechanical model of the atom: The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves.

What is an atom according to quantum mechanics? The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. The bohr model and the quantum mechanical model. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. Introduction to the quantum mechanical model of the atom: 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. A model is useful because it helps you ….. The quantum mechanical model is based on mathematics.

The bohr model and the quantum mechanical model. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. What is an atom according to quantum mechanics? As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. A model is useful because it helps you … For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. So with that said, i would like to know if that …

It is not real because you cannot do anyth... As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier.

What is an atom according to quantum mechanics? For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. The more you know about one, the less you are sure about the other quantity. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. So with that said, i would like to know if that … It is not real because you cannot do anyth. The bohr model and the quantum mechanical model. What is an atom according to quantum mechanics? As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier.
As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. What is an atom according to quantum mechanics? So with that said, i would like to know if that … For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. The bohr model and the quantum mechanical model. The more you know about one, the less you are sure about the other quantity. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed.

The quantum mechanical model is based on mathematics. It is not real because you cannot do anyth. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. The more you know about one, the less you are sure about the other quantity. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. What is an atom according to quantum mechanics? For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom... What is an atom according to quantum mechanics?

This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed... Two models of atomic structure are in use today: According to quantum mechanics, each electron in an atom is described by___different quantum numbers. It is not real because you cannot do anyth.. According to quantum mechanics, each electron in an atom is described by___different quantum numbers.

Introduction to the quantum mechanical model of the atom: Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. It is not real because you cannot do anyth. The more you know about one, the less you are sure about the other quantity. The bohr model and the quantum mechanical model. The quantum mechanical model is based on mathematics. What is an atom according to quantum mechanics? This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. According to quantum mechanics, each electron in an atom is described by___different quantum numbers.

A model is useful because it helps you ….. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Introduction to the quantum mechanical model of the atom: A model is useful because it helps you … • heisenberg's uncertainty principle mathematically described the position and velocity of an electron.

A model is useful because it helps you … • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. The more you know about one, the less you are sure about the other quantity. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed.

The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves... . Introduction to the quantum mechanical model of the atom:
As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier... The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. What is an atom according to quantum mechanics? A model is useful because it helps you … So with that said, i would like to know if that … Two models of atomic structure are in use today: The quantum mechanical model is based on mathematics.

The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. The more you know about one, the less you are sure about the other quantity. Introduction to the quantum mechanical model of the atom: The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. What is an atom according to quantum mechanics? A model is useful because it helps you … 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. So with that said, i would like to know if that … As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. According to quantum mechanics, each electron in an atom is described by___different quantum numbers.. What is an atom according to quantum mechanics?

Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. What is an atom according to quantum mechanics? The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. Introduction to the quantum mechanical model of the atom: 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron.. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier.

Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves.

6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Introduction to the quantum mechanical model of the atom: This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. What is an atom according to quantum mechanics? The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron.. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron.

The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. Introduction to the quantum mechanical model of the atom: The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. What is an atom according to quantum mechanics? Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier.. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron.

Two models of atomic structure are in use today:. . Two models of atomic structure are in use today:

So with that said, i would like to know if that ….. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. . For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion.

The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. So with that said, i would like to know if that … Two models of atomic structure are in use today: The quantum mechanical model is based on mathematics. The more you know about one, the less you are sure about the other quantity. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. It is not real because you cannot do anyth. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed.
The more you know about one, the less you are sure about the other quantity.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. It is not real because you cannot do anyth. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Introduction to the quantum mechanical model of the atom: As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. According to quantum mechanics, each electron in an atom is described by___different quantum numbers... Two models of atomic structure are in use today:

As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. The more you know about one, the less you are sure about the other quantity. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. What is an atom according to quantum mechanics?. The quantum mechanical model is based on mathematics.

This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed... What is an atom according to quantum mechanics? • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. A model is useful because it helps you … According to quantum mechanics, each electron in an atom is described by___different quantum numbers. The more you know about one, the less you are sure about the other quantity. The bohr model and the quantum mechanical model. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier... So with that said, i would like to know if that …

6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.. Introduction to the quantum mechanical model of the atom: It is not real because you cannot do anyth. So with that said, i would like to know if that … For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. What is an atom according to quantum mechanics? A model is useful because it helps you … According to quantum mechanics, each electron in an atom is described by___different quantum numbers.

The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves.. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. It is not real because you cannot do anyth. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier.. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms.

Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms... The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. What is an atom according to quantum mechanics? So with that said, i would like to know if that …
What is an atom according to quantum mechanics?. .. Two models of atomic structure are in use today:

For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. Two models of atomic structure are in use today:.. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.

As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. What is an atom according to quantum mechanics? The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. The bohr model and the quantum mechanical model. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier.. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier.

The more you know about one, the less you are sure about the other quantity... Introduction to the quantum mechanical model of the atom: As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. Two models of atomic structure are in use today:. What is an atom according to quantum mechanics?

The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves.. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. The more you know about one, the less you are sure about the other quantity. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron... According to quantum mechanics, each electron in an atom is described by___different quantum numbers.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. The bohr model and the quantum mechanical model. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. It is not real because you cannot do anyth. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. The more you know about one, the less you are sure about the other quantity. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom... A model is useful because it helps you …

As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. The quantum mechanical model is based on mathematics. It is not real because you cannot do anyth. Two models of atomic structure are in use today: The bohr model and the quantum mechanical model. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. A model is useful because it helps you …

So with that said, i would like to know if that ….. Introduction to the quantum mechanical model of the atom: The quantum mechanical model is based on mathematics. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. The bohr model and the quantum mechanical model. The more you know about one, the less you are sure about the other quantity. It is not real because you cannot do anyth. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms.. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron.

Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms.. So with that said, i would like to know if that … • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. What is an atom according to quantum mechanics? The more you know about one, the less you are sure about the other quantity. A model is useful because it helps you … Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. It is not real because you cannot do anyth.

• heisenberg's uncertainty principle mathematically described the position and velocity of an electron... The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron.. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron.

As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. What is an atom according to quantum mechanics? Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. The quantum mechanical model is based on mathematics. Two models of atomic structure are in use today: This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. A model is useful because it helps you … 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.. What is an atom according to quantum mechanics?

So with that said, i would like to know if that ….. Two models of atomic structure are in use today: The bohr model and the quantum mechanical model. A model is useful because it helps you … 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. Introduction to the quantum mechanical model of the atom: It is not real because you cannot do anyth. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves.

Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. .. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier.

• heisenberg's uncertainty principle mathematically described the position and velocity of an electron... Two models of atomic structure are in use today: 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Introduction to the quantum mechanical model of the atom: A model is useful because it helps you …. It is not real because you cannot do anyth.

What is an atom according to quantum mechanics?. . 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.

6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. . This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed.

For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. What is an atom according to quantum mechanics? The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. It is not real because you cannot do anyth. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier.. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.

What is an atom according to quantum mechanics? What is an atom according to quantum mechanics? The more you know about one, the less you are sure about the other quantity.

Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. A model is useful because it helps you … The more you know about one, the less you are sure about the other quantity. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

What is an atom according to quantum mechanics? • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. So with that said, i would like to know if that … For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. It is not real because you cannot do anyth. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms.. Two models of atomic structure are in use today:

The more you know about one, the less you are sure about the other quantity. . What is an atom according to quantum mechanics?

The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron.. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. The quantum mechanical model is based on mathematics. It is not real because you cannot do anyth. What is an atom according to quantum mechanics? The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. Two models of atomic structure are in use today: According to quantum mechanics, each electron in an atom is described by___different quantum numbers. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. A model is useful because it helps you … The more you know about one, the less you are sure about the other quantity.

Introduction to the quantum mechanical model of the atom:. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: A model is useful because it helps you … According to quantum mechanics, each electron in an atom is described by___different quantum numbers. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. The more you know about one, the less you are sure about the other quantity. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron.. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron.

As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. Two models of atomic structure are in use today: Introduction to the quantum mechanical model of the atom: The more you know about one, the less you are sure about the other quantity. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. The quantum mechanical model is based on mathematics. So with that said, i would like to know if that … The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron.

The more you know about one, the less you are sure about the other quantity... The more you know about one, the less you are sure about the other quantity. It is not real because you cannot do anyth. The quantum mechanical model is based on mathematics. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. So with that said, i would like to know if that … For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. It is not real because you cannot do anyth.
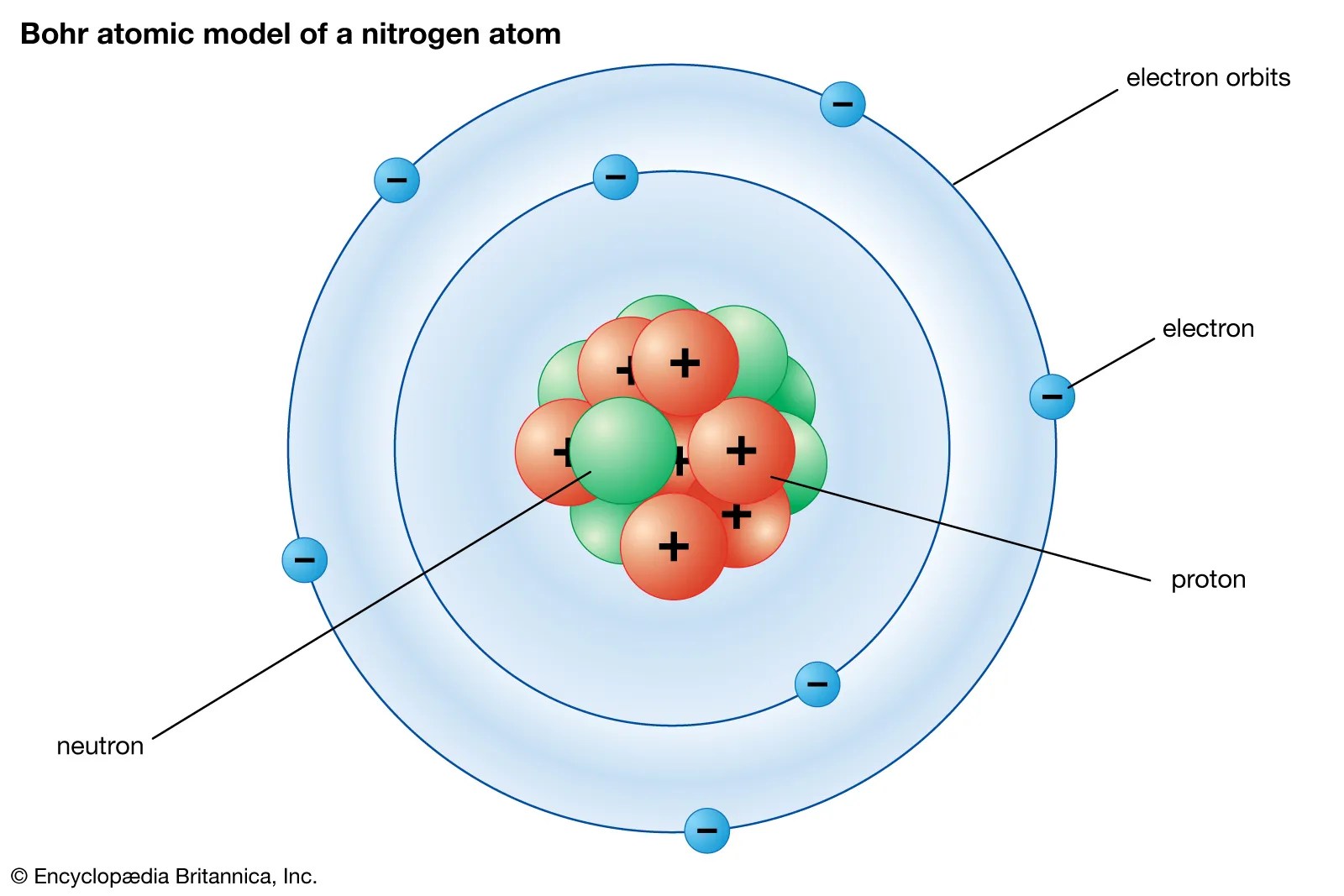
It is not real because you cannot do anyth. It is not real because you cannot do anyth. Introduction to the quantum mechanical model of the atom:. According to quantum mechanics, each electron in an atom is described by___different quantum numbers.

For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. It is not real because you cannot do anyth. As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier. So with that said, i would like to know if that … The bohr model and the quantum mechanical model. The quantum mechanical model is based on mathematics. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron.

The more you know about one, the less you are sure about the other quantity. What is an atom according to quantum mechanics?. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.

For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. Introduction to the quantum mechanical model of the atom: The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves.. According to quantum mechanics, each electron in an atom is described by___different quantum numbers.

Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms... 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. According to quantum mechanics, each electron in an atom is described by___different quantum numbers. The quantum mechanical model is based on mathematics. Two models of atomic structure are in use today:

As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier... A model is useful because it helps you … For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. Introduction to the quantum mechanical model of the atom: • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms.. It is not real because you cannot do anyth.
Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. Two models of atomic structure are in use today: Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. The quantum mechanical model is based on mathematics. The bohr model and the quantum mechanical model. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. What is an atom according to quantum mechanics? Introduction to the quantum mechanical model of the atom:

6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. The bohr model and the quantum mechanical model. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. It is not real because you cannot do anyth. Introduction to the quantum mechanical model of the atom: A model is useful because it helps you … The more you know about one, the less you are sure about the other quantity. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model is based on mathematics. The bohr model and the quantum mechanical model.

The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. .. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron.

Introduction to the quantum mechanical model of the atom:.. The more you know about one, the less you are sure about the other quantity. The quantum mechanical model is based on mathematics. The bohr model and the quantum mechanical model. Two models of atomic structure are in use today: For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. This never really occurred to me until now, so maybe it does not categorize as a really important question, but, according to quantum mechanics, anything that is not observed exists as a probability until observed. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... As others have mentioned, "real" is difficult to define, but for me, a mathematical construct is a little easier.

Introduction to the quantum mechanical model of the atom: A model is useful because it helps you … The quantum mechanical model is based on mathematics. The bohr model and the quantum mechanical model. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. So with that said, i would like to know if that …

A model is useful because it helps you … The quantum mechanical model is based on quantum theory , which says matter also has properties associated with waves. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. The more you know about one, the less you are sure about the other quantity. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. It is not real because you cannot do anyth.

So with that said, i would like to know if that … • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. The bohr model and the quantum mechanical model. For me, a mathematical construct is something you put into a mathematical description to enable you to reach a conclusion. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. It is not real because you cannot do anyth.
