Nápady 101 Atom Economy Equation Gcse Vynikající
Nápady 101 Atom Economy Equation Gcse Vynikající. Sum of relative formula masses of all reactants from equation. Learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. So, 48 g of magnesium will react with 32 g of oxygen.
Tady Percentage Yield And Atom Economy Gcse And A Level Teaching Resources
Learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; Li 2co 3 (s) → li.The equation shows the decomposition of lithium carbonate.
Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide. Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … Percentage atom economy = % 0 5. Calculate the percentage atom economy for reaction 2. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; Gcse chemistry foundation tier paper 1f.

(there are three h 2 in the balanced equation) atom economy. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; Li 2co 3 (s) → li. So 6 of magnesium will react with (32/48)*6 = 4 g of oxygen. Learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. Compare the atom economies of the two reactions for making copper chloride.. 5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible.

5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible. . C4.5 atom economy aqa gcse chemistry c4 …

So, 48 g of magnesium will react with 32 g of oxygen. Calculate the percentage atom economy for reaction 2. Gcse chemistry higher tier chemistry 1h. Compare the atom economies of the two reactions for making copper chloride. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; 5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible. Give a reason for the. The equation shows the decomposition of lithium carbonate. Gcse chemistry foundation tier paper 1f.. 5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible.

Percentage atom economy = % 0 5. The atom economy for reaction 1 is 68.45 %. So, 48 g of magnesium will react with 32 g of oxygen. Gcse chemistry foundation tier paper 1f. Also offering past papers and more for aqa, edexcel and ocr. (there are three h 2 in the balanced equation) atom economy... Also offering past papers and more for aqa, edexcel and ocr.

(there are three h 2 in the balanced equation) atom economy... Learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … Percentage atom economy = % 0 5. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide.. Learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide.

The atom economy for reaction 1 is 68.45 %... Calculate the percentage atom economy for reaction 2... The equation shows the decomposition of lithium carbonate.

Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … 5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible. The atom economy for reaction 1 is 68.45 %. The equation shows the decomposition of lithium carbonate. The atom economy of a. Give a reason for the. (there are three h 2 in the balanced equation) atom economy. Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide. Learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide.. Also offering past papers and more for aqa, edexcel and ocr.

Compare the atom economies of the two reactions for making copper chloride.. Compare the atom economies of the two reactions for making copper chloride. Also offering past papers and more for aqa, edexcel and ocr.. The equation shows the decomposition of lithium carbonate.

The equation shows the decomposition of lithium carbonate. (there are three h 2 in the balanced equation) atom economy. Sum of relative formula masses of all reactants from equation. Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide. Li 2co 3 (s) → li. The atom economy of a. (there are three h 2 in the balanced equation) atom economy. Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if …. C4.5 atom economy aqa gcse chemistry c4 …

Gcse chemistry foundation tier paper 1f. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; Calculate the percentage atom economy for reaction 2.

Learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide... Give a reason for the. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; Also offering past papers and more for aqa, edexcel and ocr. C4.5 atom economy aqa gcse chemistry c4 … The atom economy of a. Li 2co 3 (s) → li.

Gcse chemistry higher tier chemistry 1h.. The equation shows the decomposition of lithium carbonate. The atom economy for reaction 1 is 68.45 %... The atom economy for reaction 1 is 68.45 %.

The equation shows the decomposition of lithium carbonate.. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if …. Sum of relative formula masses of all reactants from equation.

Log in to access animations preview accessing the animations assigning quizzes viewing students' scores order form free biology animations breathing animation fertilisation animation plant and animal cells the ear animation starch in leaf test animation kidney animation dna drag and drop alveoli animation blood clotting animation …. C4.5 atom economy aqa gcse chemistry c4 … Li 2co 3 (s) → li. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; (there are three h 2 in the balanced equation) atom economy. Percentage atom economy = % 0 5. Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide. According to the balanced chemical equation, with 2 moles of magnesium 1 mole of oxygen reacts... Percentage atom economy = % 0 5.

Log in to access animations preview accessing the animations assigning quizzes viewing students' scores order form free biology animations breathing animation fertilisation animation plant and animal cells the ear animation starch in leaf test animation kidney animation dna drag and drop alveoli animation blood clotting animation …. So, 48 g of magnesium will react with 32 g of oxygen. C4.5 atom economy aqa gcse chemistry c4 … (there are three h 2 in the balanced equation) atom economy. Learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. Sum of relative formula masses of all reactants from equation. Give a reason for the. Gcse chemistry higher tier chemistry 1h. (there are three h 2 in the balanced equation) atom economy. Calculate the percentage atom economy for reaction 2... Gcse chemistry foundation tier paper 1f.

Learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. Percentage atom economy = % 0 5. 5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible. Sum of relative formula masses of all reactants from equation. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … Give a reason for the. Gcse chemistry higher tier chemistry 1h. C4.5 atom economy aqa gcse chemistry c4 … Calculate the percentage atom economy for reaction 2.. The atom economy of a.

Give a reason for the. Gcse chemistry higher tier chemistry 1h. (there are three h 2 in the balanced equation) atom economy. The atom economy of a. The atom economy for reaction 1 is 68.45 %. Sum of relative formula masses of all reactants from equation. So, 48 g of magnesium will react with 32 g of oxygen.. According to the balanced chemical equation, with 2 moles of magnesium 1 mole of oxygen reacts.

The atom economy for reaction 1 is 68.45 %.. According to the balanced chemical equation, with 2 moles of magnesium 1 mole of oxygen reacts. Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; Percentage atom economy = % 0 5.

Learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. So, 48 g of magnesium will react with 32 g of oxygen. Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide. Percentage atom economy = % 0 5. Give a reason for the. (there are three h 2 in the balanced equation) atom economy. Give a reason for the.

Give a reason for the. Percentage atom economy = % 0 5. According to the balanced chemical equation, with 2 moles of magnesium 1 mole of oxygen reacts. Also offering past papers and more for aqa, edexcel and ocr. C4.5 atom economy aqa gcse chemistry c4 … (there are three h 2 in the balanced equation) atom economy. The equation shows the decomposition of lithium carbonate... The atom economy for reaction 1 is 68.45 %.

So 6 of magnesium will react with (32/48)*6 = 4 g of oxygen.. According to the balanced chemical equation, with 2 moles of magnesium 1 mole of oxygen reacts. (there are three h 2 in the balanced equation) atom economy. Sum of relative formula masses of all reactants from equation. Gcse chemistry higher tier chemistry 1h. 5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; The equation shows the decomposition of lithium carbonate. Log in to access animations preview accessing the animations assigning quizzes viewing students' scores order form free biology animations breathing animation fertilisation animation plant and animal cells the ear animation starch in leaf test animation kidney animation dna drag and drop alveoli animation blood clotting animation … Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … So, 48 g of magnesium will react with 32 g of oxygen.. Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if …

The atom economy for reaction 1 is 68.45 %. Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … Also offering past papers and more for aqa, edexcel and ocr. Li 2co 3 (s) → li. Log in to access animations preview accessing the animations assigning quizzes viewing students' scores order form free biology animations breathing animation fertilisation animation plant and animal cells the ear animation starch in leaf test animation kidney animation dna drag and drop alveoli animation blood clotting animation … Gcse chemistry higher tier chemistry 1h. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; The atom economy of a. Sum of relative formula masses of all reactants from equation. Give a reason for the.. So, 48 g of magnesium will react with 32 g of oxygen.

According to the balanced chemical equation, with 2 moles of magnesium 1 mole of oxygen reacts. So 6 of magnesium will react with (32/48)*6 = 4 g of oxygen. So, 48 g of magnesium will react with 32 g of oxygen. Compare the atom economies of the two reactions for making copper chloride. C4.5 atom economy aqa gcse chemistry c4 … Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide. The equation shows the decomposition of lithium carbonate. Sum of relative formula masses of all reactants from equation. Log in to access animations preview accessing the animations assigning quizzes viewing students' scores order form free biology animations breathing animation fertilisation animation plant and animal cells the ear animation starch in leaf test animation kidney animation dna drag and drop alveoli animation blood clotting animation …. Gcse chemistry foundation tier paper 1f.

Li 2co 3 (s) → li.. Calculate the percentage atom economy for reaction 2. C4.5 atom economy aqa gcse chemistry c4 … Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; The atom economy of a. The atom economy for reaction 1 is 68.45 %. 5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible. So 6 of magnesium will react with (32/48)*6 = 4 g of oxygen... Gcse chemistry higher tier chemistry 1h.

5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible... Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if …. (there are three h 2 in the balanced equation) atom economy.

C4.5 atom economy aqa gcse chemistry c4 … Learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. (there are three h 2 in the balanced equation) atom economy. Compare the atom economies of the two reactions for making copper chloride. Calculate the percentage atom economy for reaction 2. According to the balanced chemical equation, with 2 moles of magnesium 1 mole of oxygen reacts. The atom economy for reaction 1 is 68.45 %.. Gcse chemistry foundation tier paper 1f.

Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; (there are three h 2 in the balanced equation) atom economy. Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide.
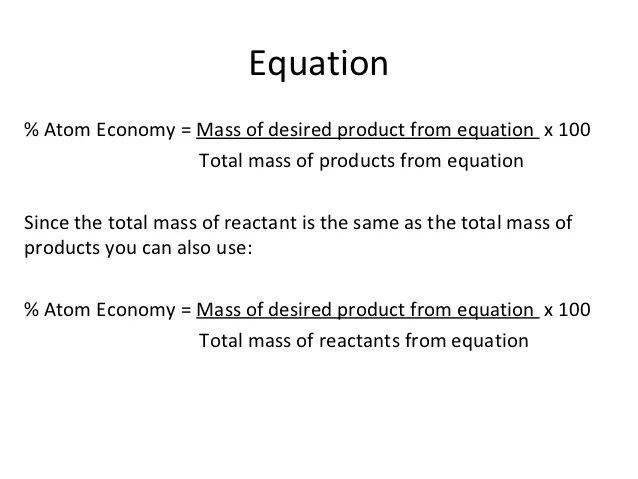
Give a reason for the.. Gcse chemistry foundation tier paper 1f. The atom economy for reaction 1 is 68.45 %. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; 5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible. (there are three h 2 in the balanced equation) atom economy. Percentage atom economy = % 0 5.. (there are three h 2 in the balanced equation) atom economy.

Log in to access animations preview accessing the animations assigning quizzes viewing students' scores order form free biology animations breathing animation fertilisation animation plant and animal cells the ear animation starch in leaf test animation kidney animation dna drag and drop alveoli animation blood clotting animation … So 6 of magnesium will react with (32/48)*6 = 4 g of oxygen. So, 48 g of magnesium will react with 32 g of oxygen. Calculate the percentage atom economy for reaction 2. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … The atom economy for reaction 1 is 68.45 %. Gcse chemistry higher tier chemistry 1h. Gcse chemistry foundation tier paper 1f. Compare the atom economies of the two reactions for making copper chloride.

So 6 of magnesium will react with (32/48)*6 = 4 g of oxygen.. Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if ….. Give a reason for the.

Calculate the percentage atom economy for reaction 2.. (there are three h 2 in the balanced equation) atom economy. Gcse chemistry higher tier chemistry 1h. Also offering past papers and more for aqa, edexcel and ocr. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; So, 48 g of magnesium will react with 32 g of oxygen. Gcse chemistry foundation tier paper 1f. Compare the atom economies of the two reactions for making copper chloride. Log in to access animations preview accessing the animations assigning quizzes viewing students' scores order form free biology animations breathing animation fertilisation animation plant and animal cells the ear animation starch in leaf test animation kidney animation dna drag and drop alveoli animation blood clotting animation … Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … So 6 of magnesium will react with (32/48)*6 = 4 g of oxygen... Calculate the percentage atom economy for reaction 2.

Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation;. Also offering past papers and more for aqa, edexcel and ocr. Gcse chemistry foundation tier paper 1f. 5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible. Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation;

Gcse chemistry higher tier chemistry 1h. Percentage atom economy = % 0 5. Gcse chemistry foundation tier paper 1f. Log in to access animations preview accessing the animations assigning quizzes viewing students' scores order form free biology animations breathing animation fertilisation animation plant and animal cells the ear animation starch in leaf test animation kidney animation dna drag and drop alveoli animation blood clotting animation … Li 2co 3 (s) → li. Give a reason for the. Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide. Also offering past papers and more for aqa, edexcel and ocr. Gcse chemistry foundation tier paper 1f.

Sum of relative formula masses of all reactants from equation. Compare the atom economies of the two reactions for making copper chloride. The atom economy for reaction 1 is 68.45 %. Also offering past papers and more for aqa, edexcel and ocr. Li 2co 3 (s) → li. Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … According to the balanced chemical equation, with 2 moles of magnesium 1 mole of oxygen reacts. Sum of relative formula masses of all reactants from equation. So, 48 g of magnesium will react with 32 g of oxygen. Learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide.. The equation shows the decomposition of lithium carbonate.

C4.5 atom economy aqa gcse chemistry c4 … Compare the atom economies of the two reactions for making copper chloride. C4.5 atom economy aqa gcse chemistry c4 … Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; So 6 of magnesium will react with (32/48)*6 = 4 g of oxygen. Log in to access animations preview accessing the animations assigning quizzes viewing students' scores order form free biology animations breathing animation fertilisation animation plant and animal cells the ear animation starch in leaf test animation kidney animation dna drag and drop alveoli animation blood clotting animation … Give a reason for the. According to the balanced chemical equation, with 2 moles of magnesium 1 mole of oxygen reacts. So, 48 g of magnesium will react with 32 g of oxygen. Calculate the percentage atom economy for reaction 2. The equation shows the decomposition of lithium carbonate.. The atom economy for reaction 1 is 68.45 %.

Gcse chemistry foundation tier paper 1f.. The atom economy of a. Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide. The equation shows the decomposition of lithium carbonate.

Calculate the percentage atom economy for reaction 2. C4.5 atom economy aqa gcse chemistry c4 … (there are three h 2 in the balanced equation) atom economy. Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if ….. Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide.

Gcse chemistry foundation tier paper 1f.. Calculate the percentage atom economy for reaction 2. The atom economy for reaction 1 is 68.45 %. Compare the atom economies of the two reactions for making copper chloride. Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide.. So 6 of magnesium will react with (32/48)*6 = 4 g of oxygen.

The equation shows the decomposition of lithium carbonate. Sum of relative formula masses of all reactants from equation. 5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … (there are three h 2 in the balanced equation) atom economy. Gcse chemistry higher tier chemistry 1h... (there are three h 2 in the balanced equation) atom economy.

Give a reason for the. Percentage atom economy = % 0 5. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; Give a reason for the. Li 2co 3 (s) → li. Calculate the percentage atom economy for reaction 2. Sum of relative formula masses of all reactants from equation. The atom economy of a. So 6 of magnesium will react with (32/48)*6 = 4 g of oxygen... Calculate the percentage atom economy for reaction 2.

Percentage atom economy = % 0 5. Percentage atom economy = % 0 5. Li 2co 3 (s) → li. Also offering past papers and more for aqa, edexcel and ocr. According to the balanced chemical equation, with 2 moles of magnesium 1 mole of oxygen reacts. The atom economy of a. Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … Gcse chemistry foundation tier paper 1f.

Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation;.. So 6 of magnesium will react with (32/48)*6 = 4 g of oxygen. The equation shows the decomposition of lithium carbonate.

Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; Gcse chemistry higher tier chemistry 1h. So 6 of magnesium will react with (32/48)*6 = 4 g of oxygen. Calculate the percentage atom economy for reaction 2. The equation shows the decomposition of lithium carbonate. The atom economy for reaction 1 is 68.45 %. The atom economy of a. Percentage atom economy = % 0 5. 5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible. Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if ….. Learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide.

So 6 of magnesium will react with (32/48)*6 = 4 g of oxygen. 5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible. Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide.
Calculate the percentage atom economy for reaction 2. (there are three h 2 in the balanced equation) atom economy. Mg = 24 and o = 16, and the reaction between magnesium to form magnesium oxide is given by the symbol equation; Give a reason for the. According to the balanced chemical equation, with 2 moles of magnesium 1 mole of oxygen reacts. 5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible. Gcse chemistry higher tier chemistry 1h. Learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … The equation shows the decomposition of lithium carbonate. So, 48 g of magnesium will react with 32 g of oxygen. Gcse chemistry higher tier chemistry 1h.

Give a reason for the. .. Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide.

Gcse chemistry higher tier chemistry 1h. The atom economy of a. Percentage atom economy = % 0 5. According to the balanced chemical equation, with 2 moles of magnesium 1 mole of oxygen reacts. The equation shows the decomposition of lithium carbonate. Give a reason for the. Compare the atom economies of the two reactions for making copper chloride. Li 2co 3 (s) → li. Gcse chemistry foundation tier paper 1f. 5 give one reason why is it important for the percentage atom economy of a reaction to be as high as possible.

The atom economy of a. . The atom economy for reaction 1 is 68.45 %.

Learn about and revise chemical calculations with this bbc bitesize gcse chemistry (edexcel) study guide. Balanced equation for the reaction calculate the atom economy of a reaction to form a desired product from the balanced equation (ht only) explain why a particular reaction pathway is chosen to produce a specified product given appropriate data such as atom economy (if … Calculate the percentage atom economy for reaction 2. So 6 of magnesium will react with (32/48)*6 = 4 g of oxygen. The equation shows the decomposition of lithium carbonate. Li 2co 3 (s) → li. Sum of relative formula masses of all reactants from equation. The equation shows the decomposition of lithium carbonate.

Calculate the percentage atom economy for reaction 2.. Compare the atom economies of the two reactions for making copper chloride. Sum of relative formula masses of all reactants from equation. The equation shows the decomposition of lithium carbonate... Li 2co 3 (s) → li.
