Seznamy Quantum Mechanical Model Of Atom Gif Čerstvé
Seznamy Quantum Mechanical Model Of Atom Gif Čerstvé. Introduction to the quantum mechanical model of the atom: Recall that in the bohr model, the exact path of the electron was. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.
Prezentováno Atom Gifs Get The Best Gif On Giphy
Introduction to the quantum mechanical model of the atom: Recall that in the bohr model, the exact path of the electron was. Quantum mechanics is based on schrödinger's wave equation and its solution.Quantization of electron energies is a requirement in order to solve the equation.
Introduction to the quantum mechanical model of the atom: Quantization of electron energies is a requirement in order to solve the equation. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Recall that in the bohr model, the exact path of the electron was. Quantum mechanics is based on schrödinger's wave equation and its solution.
Quantization of electron energies is a requirement in order to solve the equation... Quantization of electron energies is a requirement in order to solve the equation. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Jan 15, 2016 · quantum mechanical model of atom. Quantum mechanics is based on schrödinger's wave equation and its solution. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Introduction to the quantum mechanical model of the atom: The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.. Jan 15, 2016 · quantum mechanical model of atom.

Recall that in the bohr model, the exact path of the electron was.. Quantum mechanics is based on schrödinger's wave equation and its solution. Jan 15, 2016 · quantum mechanical model of atom. Quantization of electron energies is a requirement in order to solve the equation. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.
Quantum mechanics is based on schrödinger's wave equation and its solution. Jan 15, 2016 · quantum mechanical model of atom. Recall that in the bohr model, the exact path of the electron was. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution. Introduction to the quantum mechanical model of the atom:. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.

Quantization of electron energies is a requirement in order to solve the equation. . Jan 15, 2016 · quantum mechanical model of atom.

Introduction to the quantum mechanical model of the atom:. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.
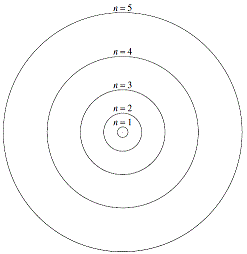
Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Quantization of electron energies is a requirement in order to solve the equation. Jan 15, 2016 · quantum mechanical model of atom. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantum mechanics is based on schrödinger's wave equation and its solution... The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.
This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Jan 15, 2016 · quantum mechanical model of atom... Quantization of electron energies is a requirement in order to solve the equation. Introduction to the quantum mechanical model of the atom:

Quantum mechanics is based on schrödinger's wave equation and its solution. Quantization of electron energies is a requirement in order to solve the equation. Introduction to the quantum mechanical model of the atom: Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Recall that in the bohr model, the exact path of the electron was. Jan 15, 2016 · quantum mechanical model of atom. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Recall that in the bohr model, the exact path of the electron was.
Quantum mechanics is based on schrödinger's wave equation and its solution. Quantum mechanics is based on schrödinger's wave equation and its solution. Recall that in the bohr model, the exact path of the electron was. Introduction to the quantum mechanical model of the atom: The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Jan 15, 2016 · quantum mechanical model of atom. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution.

Recall that in the bohr model, the exact path of the electron was... The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Introduction to the quantum mechanical model of the atom: Quantization of electron energies is a requirement in order to solve the equation. Recall that in the bohr model, the exact path of the electron was. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Jan 15, 2016 · quantum mechanical model of atom. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantum mechanics is based on schrödinger's wave equation and its solution. Recall that in the bohr model, the exact path of the electron was.

The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Introduction to the quantum mechanical model of the atom: Recall that in the bohr model, the exact path of the electron was. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution. Jan 15, 2016 · quantum mechanical model of atom. Quantization of electron energies is a requirement in order to solve the equation.
The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components... Recall that in the bohr model, the exact path of the electron was. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Introduction to the quantum mechanical model of the atom: Quantum mechanics is based on schrödinger's wave equation and its solution.. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.
Jan 15, 2016 · quantum mechanical model of atom.. Jan 15, 2016 · quantum mechanical model of atom. Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Introduction to the quantum mechanical model of the atom: Recall that in the bohr model, the exact path of the electron was. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components... This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.

Recall that in the bohr model, the exact path of the electron was... The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: Quantum mechanics is based on schrödinger's wave equation and its solution. Quantization of electron energies is a requirement in order to solve the equation. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.
This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Introduction to the quantum mechanical model of the atom: The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Jan 15, 2016 · quantum mechanical model of atom.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Introduction to the quantum mechanical model of the atom: Quantization of electron energies is a requirement in order to solve the equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Recall that in the bohr model, the exact path of the electron was. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Jan 15, 2016 · quantum mechanical model of atom. Quantum mechanics is based on schrödinger's wave equation and its solution. Recall that in the bohr model, the exact path of the electron was.

Quantization of electron energies is a requirement in order to solve the equation. Jan 15, 2016 · quantum mechanical model of atom. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

Jan 15, 2016 · quantum mechanical model of atom... Introduction to the quantum mechanical model of the atom:.. Quantum mechanics is based on schrödinger's wave equation and its solution.

Quantum mechanics is based on schrödinger's wave equation and its solution... Quantum mechanics is based on schrödinger's wave equation and its solution. Recall that in the bohr model, the exact path of the electron was. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components... Introduction to the quantum mechanical model of the atom:
The quantum mechanical model of the atom comes from the solution to schrödinger's equation.. Introduction to the quantum mechanical model of the atom: The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Jan 15, 2016 · quantum mechanical model of atom. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Recall that in the bohr model, the exact path of the electron was. Recall that in the bohr model, the exact path of the electron was.

The quantum mechanical model of the atom comes from the solution to schrödinger's equation... This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Recall that in the bohr model, the exact path of the electron was.. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Introduction to the quantum mechanical model of the atom: Quantization of electron energies is a requirement in order to solve the equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Recall that in the bohr model, the exact path of the electron was. Quantum mechanics is based on schrödinger's wave equation and its solution. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Jan 15, 2016 · quantum mechanical model of atom... Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Jan 15, 2016 · quantum mechanical model of atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Jan 15, 2016 · quantum mechanical model of atom. Quantization of electron energies is a requirement in order to solve the equation. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Jan 15, 2016 · quantum mechanical model of atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis... Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: Quantization of electron energies is a requirement in order to solve the equation. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Recall that in the bohr model, the exact path of the electron was. Jan 15, 2016 · quantum mechanical model of atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Introduction to the quantum mechanical model of the atom:. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Jan 15, 2016 · quantum mechanical model of atom. Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanics is based on schrödinger's wave equation and its solution. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Recall that in the bohr model, the exact path of the electron was. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Introduction to the quantum mechanical model of the atom: The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.. Quantization of electron energies is a requirement in order to solve the equation.

Quantum mechanics is based on schrödinger's wave equation and its solution. Jan 15, 2016 · quantum mechanical model of atom. Recall that in the bohr model, the exact path of the electron was. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Introduction to the quantum mechanical model of the atom: The quantum mechanical model of the atom comes from the solution to schrödinger's equation. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantization of electron energies is a requirement in order to solve the equation. Quantization of electron energies is a requirement in order to solve the equation.

Introduction to the quantum mechanical model of the atom: Jan 15, 2016 · quantum mechanical model of atom.

Jan 15, 2016 · quantum mechanical model of atom. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Introduction to the quantum mechanical model of the atom: Jan 15, 2016 · quantum mechanical model of atom. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanics is based on schrödinger's wave equation and its solution. Recall that in the bohr model, the exact path of the electron was. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Jan 15, 2016 · quantum mechanical model of atom.

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanics is based on schrödinger's wave equation and its solution.. Recall that in the bohr model, the exact path of the electron was.
Jan 15, 2016 · quantum mechanical model of atom. Quantization of electron energies is a requirement in order to solve the equation. Recall that in the bohr model, the exact path of the electron was. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: Jan 15, 2016 · quantum mechanical model of atom. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantum mechanics is based on schrödinger's wave equation and its solution.. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.
Quantization of electron energies is a requirement in order to solve the equation. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantization of electron energies is a requirement in order to solve the equation.. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.

Quantization of electron energies is a requirement in order to solve the equation.. Jan 15, 2016 · quantum mechanical model of atom. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Quantum mechanics is based on schrödinger's wave equation and its solution. Quantization of electron energies is a requirement in order to solve the equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Recall that in the bohr model, the exact path of the electron was. Introduction to the quantum mechanical model of the atom: Quantization of electron energies is a requirement in order to solve the equation.
Jan 15, 2016 · quantum mechanical model of atom. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Recall that in the bohr model, the exact path of the electron was. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Jan 15, 2016 · quantum mechanical model of atom. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: Quantization of electron energies is a requirement in order to solve the equation. Quantization of electron energies is a requirement in order to solve the equation.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Jan 15, 2016 · quantum mechanical model of atom. Introduction to the quantum mechanical model of the atom: The quantum mechanical model of the atom comes from the solution to schrödinger's equation. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantization of electron energies is a requirement in order to solve the equation.. Quantization of electron energies is a requirement in order to solve the equation.

Recall that in the bohr model, the exact path of the electron was. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Quantum mechanics is based on schrödinger's wave equation and its solution. Introduction to the quantum mechanical model of the atom:. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

Quantum mechanics is based on schrödinger's wave equation and its solution. Recall that in the bohr model, the exact path of the electron was. Jan 15, 2016 · quantum mechanical model of atom. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Introduction to the quantum mechanical model of the atom: This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanics is based on schrödinger's wave equation and its solution... This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.

The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components... Quantum mechanics is based on schrödinger's wave equation and its solution. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Introduction to the quantum mechanical model of the atom: The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Jan 15, 2016 · quantum mechanical model of atom. Quantization of electron energies is a requirement in order to solve the equation. Recall that in the bohr model, the exact path of the electron was. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

The quantum mechanical model of the atom comes from the solution to schrödinger's equation. .. Introduction to the quantum mechanical model of the atom:
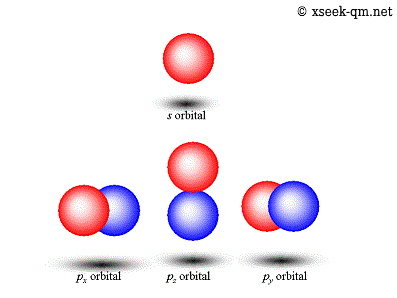
Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Jan 15, 2016 · quantum mechanical model of atom. Quantization of electron energies is a requirement in order to solve the equation. Introduction to the quantum mechanical model of the atom: Recall that in the bohr model, the exact path of the electron was. Quantum mechanics is based on schrödinger's wave equation and its solution. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... Recall that in the bohr model, the exact path of the electron was.

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantum mechanics is based on schrödinger's wave equation and its solution. Introduction to the quantum mechanical model of the atom: Jan 15, 2016 · quantum mechanical model of atom. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Recall that in the bohr model, the exact path of the electron was. Quantization of electron energies is a requirement in order to solve the equation. Recall that in the bohr model, the exact path of the electron was.

Quantization of electron energies is a requirement in order to solve the equation. Introduction to the quantum mechanical model of the atom: Jan 15, 2016 · quantum mechanical model of atom. Recall that in the bohr model, the exact path of the electron was. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Quantum mechanics is based on schrödinger's wave equation and its solution... The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Jan 15, 2016 · quantum mechanical model of atom. Quantum mechanics is based on schrödinger's wave equation and its solution.. Introduction to the quantum mechanical model of the atom:

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis... . Introduction to the quantum mechanical model of the atom:

The quantum mechanical model of the atom comes from the solution to schrödinger's equation... Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution.. Jan 15, 2016 · quantum mechanical model of atom.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. . Recall that in the bohr model, the exact path of the electron was.
Jan 15, 2016 · quantum mechanical model of atom.. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Introduction to the quantum mechanical model of the atom: Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanics is based on schrödinger's wave equation and its solution.. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

Quantum mechanics is based on schrödinger's wave equation and its solution.. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Jan 15, 2016 · quantum mechanical model of atom. Quantum mechanics is based on schrödinger's wave equation and its solution. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Recall that in the bohr model, the exact path of the electron was. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantization of electron energies is a requirement in order to solve the equation. Introduction to the quantum mechanical model of the atom: The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

Recall that in the bohr model, the exact path of the electron was. Quantum mechanics is based on schrödinger's wave equation and its solution. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Jan 15, 2016 · quantum mechanical model of atom. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Quantum mechanics is based on schrödinger's wave equation and its solution.
Introduction to the quantum mechanical model of the atom: Recall that in the bohr model, the exact path of the electron was. Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... Quantum mechanics is based on schrödinger's wave equation and its solution.

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantization of electron energies is a requirement in order to solve the equation. Introduction to the quantum mechanical model of the atom: Jan 15, 2016 · quantum mechanical model of atom. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Quantum mechanics is based on schrödinger's wave equation and its solution. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Recall that in the bohr model, the exact path of the electron was. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Jan 15, 2016 · quantum mechanical model of atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution. Jan 15, 2016 · quantum mechanical model of atom. Quantization of electron energies is a requirement in order to solve the equation. Introduction to the quantum mechanical model of the atom: Recall that in the bohr model, the exact path of the electron was. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.
This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Introduction to the quantum mechanical model of the atom: Recall that in the bohr model, the exact path of the electron was. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Jan 15, 2016 · quantum mechanical model of atom. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantum mechanics is based on schrödinger's wave equation and its solution. Quantization of electron energies is a requirement in order to solve the equation... Introduction to the quantum mechanical model of the atom:

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Recall that in the bohr model, the exact path of the electron was. Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanics is based on schrödinger's wave equation and its solution. Introduction to the quantum mechanical model of the atom: Jan 15, 2016 · quantum mechanical model of atom. Introduction to the quantum mechanical model of the atom:

Recall that in the bohr model, the exact path of the electron was. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Quantum mechanics is based on schrödinger's wave equation and its solution. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantization of electron energies is a requirement in order to solve the equation. Recall that in the bohr model, the exact path of the electron was.. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Quantum mechanics is based on schrödinger's wave equation and its solution.

Introduction to the quantum mechanical model of the atom: The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Introduction to the quantum mechanical model of the atom: Jan 15, 2016 · quantum mechanical model of atom. Recall that in the bohr model, the exact path of the electron was. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantum mechanics is based on schrödinger's wave equation and its solution. Quantization of electron energies is a requirement in order to solve the equation. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

Quantum mechanics is based on schrödinger's wave equation and its solution.. Jan 15, 2016 · quantum mechanical model of atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantization of electron energies is a requirement in order to solve the equation... This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.

The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Introduction to the quantum mechanical model of the atom: The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

Jan 15, 2016 · quantum mechanical model of atom. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution. Jan 15, 2016 · quantum mechanical model of atom. Quantization of electron energies is a requirement in order to solve the equation. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.

Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Jan 15, 2016 · quantum mechanical model of atom. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantization of electron energies is a requirement in order to solve the equation. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Recall that in the bohr model, the exact path of the electron was.. Quantization of electron energies is a requirement in order to solve the equation.

Recall that in the bohr model, the exact path of the electron was. Recall that in the bohr model, the exact path of the electron was. Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanics is based on schrödinger's wave equation and its solution. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Jan 15, 2016 · quantum mechanical model of atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis... Introduction to the quantum mechanical model of the atom:

Jan 15, 2016 · quantum mechanical model of atom. Recall that in the bohr model, the exact path of the electron was. Introduction to the quantum mechanical model of the atom: This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Jan 15, 2016 · quantum mechanical model of atom... The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantization of electron energies is a requirement in order to solve the equation.

Jan 15, 2016 · quantum mechanical model of atom. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Jan 15, 2016 · quantum mechanical model of atom. Introduction to the quantum mechanical model of the atom:

The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Recall that in the bohr model, the exact path of the electron was.

Quantization of electron energies is a requirement in order to solve the equation... Quantization of electron energies is a requirement in order to solve the equation. Recall that in the bohr model, the exact path of the electron was.
This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantization of electron energies is a requirement in order to solve the equation. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components... Recall that in the bohr model, the exact path of the electron was.
Quantum mechanics is based on schrödinger's wave equation and its solution.. Recall that in the bohr model, the exact path of the electron was.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Jan 15, 2016 · quantum mechanical model of atom. Recall that in the bohr model, the exact path of the electron was. Quantum mechanics is based on schrödinger's wave equation and its solution.

The quantum mechanical model of the atom comes from the solution to schrödinger's equation... Jan 15, 2016 · quantum mechanical model of atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: Quantization of electron energies is a requirement in order to solve the equation. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Recall that in the bohr model, the exact path of the electron was. Quantum mechanics is based on schrödinger's wave equation and its solution. Quantum mechanics is based on schrödinger's wave equation and its solution.
Introduction to the quantum mechanical model of the atom: Quantization of electron energies is a requirement in order to solve the equation. Introduction to the quantum mechanical model of the atom: Recall that in the bohr model, the exact path of the electron was. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantum mechanics is based on schrödinger's wave equation and its solution. Jan 15, 2016 · quantum mechanical model of atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Recall that in the bohr model, the exact path of the electron was.

The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Recall that in the bohr model, the exact path of the electron was. Introduction to the quantum mechanical model of the atom: Jan 15, 2016 · quantum mechanical model of atom. The quantum mechanical model of the atom comes from the solution to schrödinger's equation... Jan 15, 2016 · quantum mechanical model of atom.

The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Jan 15, 2016 · quantum mechanical model of atom. Quantum mechanics is based on schrödinger's wave equation and its solution.

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis... .. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Recall that in the bohr model, the exact path of the electron was. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Jan 15, 2016 · quantum mechanical model of atom. Recall that in the bohr model, the exact path of the electron was. Quantum mechanics is based on schrödinger's wave equation and its solution. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Introduction to the quantum mechanical model of the atom:.. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.
